Bio pharmaceutical Business Unit
NEURONATA-R®
NEURONATA-R® inj. (Autologous BM-MSC) is an autologous bone marrow
mesenchymal stem cell (autologous BM-MSC) therapy that acts as a neuroprotective effect
and relieves progression of disease through prevention of motor nerve cell, survival extension
of motor neurons, releasing nerves’ inflammatory and immune regulation function.
-
Overview of NEURONATA-R
Neuronata-R inj : Neuro “Nerve” + Nata “Birth”
-
Type
Orphan drug
-
Approval
2014. 07. 30 (MFDS)
-
Generic Name
Lenzumestrocel
-
Dosage
1.0 x 106 cells/ kg of body weight concomitant with riluzole
-
Administration
Intrathecal administration, two injections with 4 weeks interval
-
Effect & Efficacy
Delaying of ALS progression
-
Characteristics
White suspension fluid filled inside a transparent colorless plastic syringe
-
Amount
4.0 x 107 cells /4mL, pre-filled syringe
-
Storage
Cold storage (2~8℃)
-
Expiration Date
48 hours after manufacturing
Introduction to Clinical Results
NeuroNata-R is an autologous bone marrow-derived stem cell therapy for the treatment of amyotrophic lateral sclerosis (ALS).
NeuroNata-R secretes various growth factors, immune modulators, and anti-inflammatory factors, which contribute to the alleviation of neuroinflammation,
prevention of motor neuron cell death, extension of survival, and neuroprotective effects.
As a result, it helps alleviate the progression rate of amyotrophic lateral sclerosis (ALS) as measured by the ALS Functional Rating Scale-Revised (ALSFRS-R*).
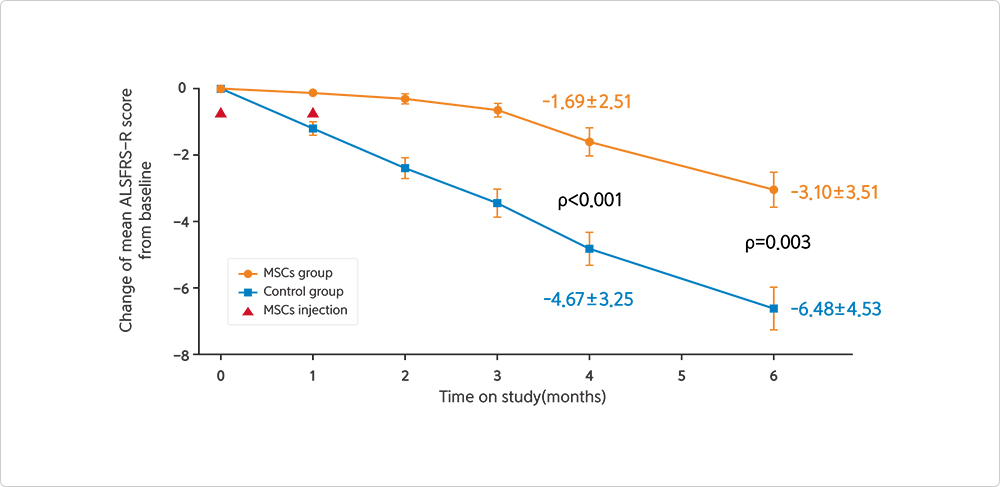
Change of ALSFRS-R Score after NEURONATA-R® Treatment (Phase II)
- Data analysis was conducted on a total of 59 ALS patients (MSCs group: 32 patients, CONTROL group: 27 patients) in a phase 2 clinical trial.
- Four months after the procedure, there was a significant difference in the ALSFRS-R score between the MSCs and CONTROL groups, with a difference of 2.98 points (MSCs group score change: -1.69±2.51, CONTROL group score change: -4.67±3.25, P < 0.001). Additionally, at six months after the procedure, there was a difference of 3.38 points in the ALSFRS-R score (MSCs group score change: -3.10±3.51, CONTROL group score change: -6.48±4.53, P=0.003).
- ALSFRS-R : Amyotrophic Lateral Sclerosis Functional Rating Scale-Revised
- MSCs : Mesenchymal Stem Cells
MoA of NEURONATA-R® in ALS
NEURONATA-R® derived from mesenchymal stem cell
in bone marrow produces anti-inflammatory cytokines dominant for neuroprotective effects on motor neurons.
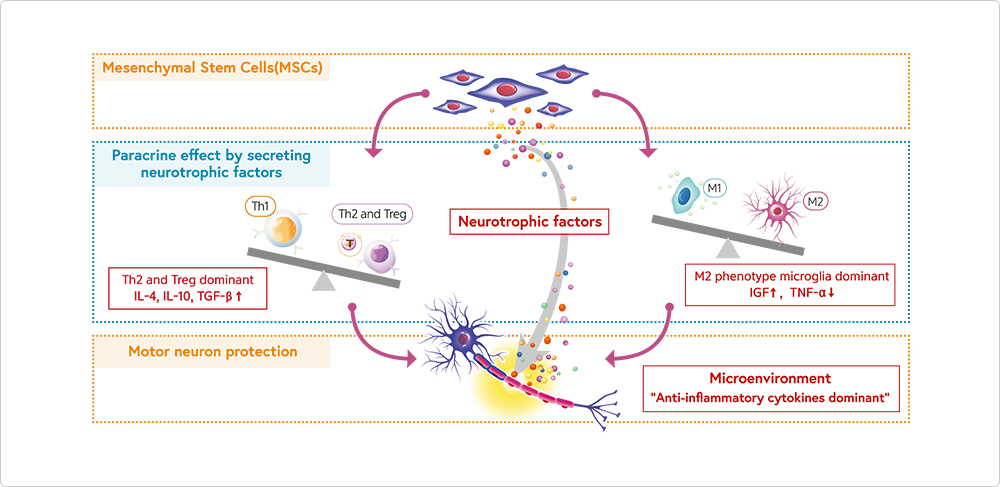
- Treatment with MSCs increases population ratio of Th2 and Treg cells by producing anti-inflammatory factors such as IL-4, IL-10 and TGF-β.
- Treatment with MSCs increases M2 phenotype by suppressing TNF-α and IL-1β.
- Treatment with MSCs has paracrine effects by secreting neurotropic factors such as BDNF, VEGF and IGF which have protective effects on motor neurons.
- The mechanism of NeuroNata-R therapy in ALS is 1) T-regulatory cell induction, 2) Th2 cell activation, 3) Increased conversion of M2 population, 4) Increased anti-inflammatory cytokines, 5) Released neurotropic factor including VEGF, NGF, BNDF
- Effectiveness of bone marrow stem cell was mediated by immune-inflammation modulation by elevating the T-regulatory cell population and converting microglial cells from M1 to M2 phenotype
- Repeated intrathecal injections of autologous MSC was safe and effective for delaying the progression speed of ALS
- Ref 1.Phase I trial of repeated intrathecal autologous bone marrow-derived Mes
enchymal stromal cells in
amyotrophic lateral sclerosis. Stem Cell Tansl Med, 2015 Jun;4(6):590-7 - Ref 2.Endogenous regulatory T lymphocytes ameliorate amyotrophic lateral sclerosis in
mice and correlate with disease
progression in patients with amyotrophic lateral sclerosis. Brain 2011:134;1293-1314 - Ref 3.Repeated intrathecal mesenchymal stem cells for amyotrophic lateral sclerosis.
Annals of Neurology, 26 July 2018;
Online published
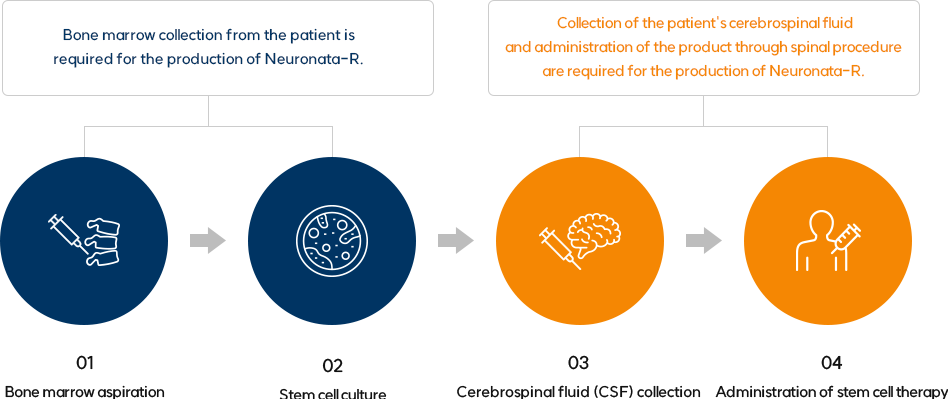
Treatment Method
Neuronata-R consists of ingredients that are pharmaceuticals derived from
autologous bone marrow mesenchymal stem cells and autologous cerebrospinal fluid
To produce the pharmaceutical, collection of the patient's bone marrow and cerebrospinal fluid is necessary.
Neuronata-R is a pharmaceutical treatment accompanied by a spinal procedure for administration.
| Category | Content |
|---|---|
| Name of Product | NEURONATA-R® |
| Amount | 4.0×107cells / 4mL, pre-filled syringe |
| Characteristics | White suspension fluid filled inside a transparent colorless plastic syringe. |
| Effect & Efficacy | Delaying of ALS progression |
| Dosage | 1.0 x 106 cells/ kg of body weight concomitant with riluzole |
| Approval | 2014. 07. 30 (MFDS) |
| Specialized/General | Specialty medicine, Orphan drugs |
| Developer/Manufacturer | CORESTEMCHEMON Inc. |

